Frequently Asked Questions
What is a brand name drug?
• A brand name drug is the first version of a drug to be sold within a country.
• A brand name drug is sold by the drug manufacturer that first researched and developed the drug.
What is a generic drug?
• A generic drug is a legal copy of a brand name drug.
How are generic and brand name drugs the same?
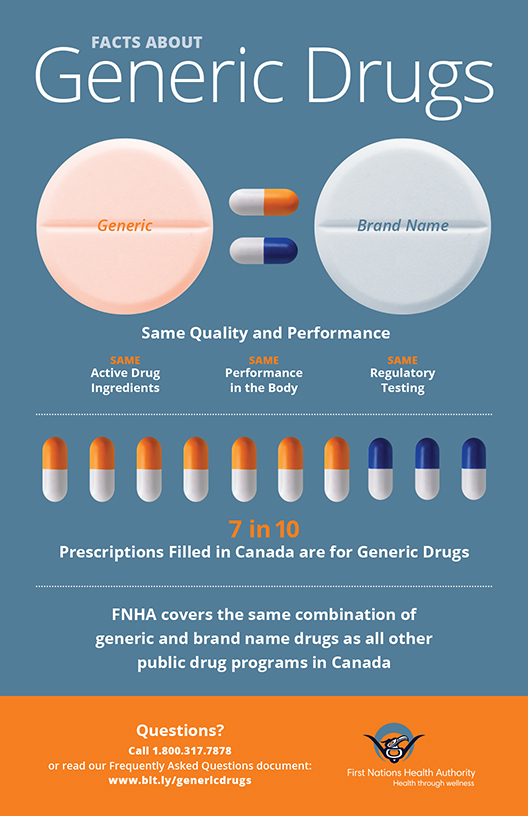
• They both have the same active ingredient (the compound that makes the drug work).
• They both have the same amount of the active ingredient.
• They both work the same way in the body.
• They are both absorbed by the body at the same rate.
How are generic and brand name drugs different?
• They may have different inactive ingredients (such as flavours or preservatives).
• They may have slightly different colours, shapes, or markings.
• Brand name drugs are more expensive.
Why are brand name drugs more expensive than generic drugs?
• Brand name drugs are more expensive because of the large investments brand name manufacturers make in marketing, branding, research and development (R&D). These investments increase prices by 30 to 40 percent.
• Generic drug manufacturers do not invest in R&D, marketing or branding the same way brand name manufacturers do. With minimal marketing and R&D expenses, the generic drug price is 30 to 40 per cent lower.
Are brand name drugs safer? How do I know if a generic drug is safe?
• Generics are just as safe as brand name drugs. Both generic and brand name drugs are rigorously tested by Health Canada for quality, safety and effectiveness.
• Health Canada also has regulations for generic drug manufacturing. All drug manufacturers in Canada must follow the same regulations for their manufacturing processes and for ensuring the quality of their ingredients.
Are generic drugs as effective as brand name drugs?
Yes. Health Canada reviews and approves all drugs before they can be sold in Canada. For generic drugs, studies must show that generic drug formulations have been thoroughly tested to ensure the same rate and extent of absorption by the body as brand name drugs. Testing also proves that the active ingredient reaches the same concentration in the blood in the same amount of time as the brand name drug.
Are generic drugs as good as brand name drugs?
Yes. Generic drugs are high-quality medicine. A generic drug is a legal copy of the brand name drug.
Do generic drugs contain the same medicine as brand name drugs?
Yes. Both generic and brand name drugs contain the same active medicinal ingredients.
How are drugs researched and approved for sale in Canada?
A brand name drug manufacturer spends many years researching and developing a new drug before it can be approved for sale in Canada. The drug manufacturer applies for a patent on the drug so that no other company can manufacture or sell it.
Once it has a patent, the brand name manufacturer makes large investments in marketing and branding campaigns to sell its new drug. When the patent expires (usually after ten years), all other drug manufacturers are legally allowed to make copies of the brand name drug and sell it as a 'generic drug'.
Are generic drugs given to BC First Nations more often?
No.
• The same generic and brand name drugs are prescribed for the same conditions to both First Nations and non-First Nations individuals.
• The FNHA prescription drug list is similar to the BC Pharmacare prescription drug list that is mandated for all citizens of BC.
• Generic drugs are commonly used by all Canadians. Prescriptions in Canada are usually filled with generic drugs if they are available.
Since transfer, has FNHA made changes to generic or brand name coverage?
No. BC First Nations receive the same proportion of generic and brand names drugs as before transfer. Most drug plans – including Pharmacare, Veterans and NIHB – switch coverage to the generic version of a drug once the patent on the brand name drug has expired.
Why are there changes in generic brand coverage?
Each year in April, BC Pharmacare selects which generic brands it will cover based on price and the ability to supply all BC residents (and prevent drug shortages). Pharmacies often switch the generic brands they carry in the spring because of this change in BC Pharmacare coverage.
When might it be unsafe to switch to a generic brand?
Rarely someone might be allergic to an in-active ingredient in a drug. If you are allergic to ingredients such as lactose, gluten, sulfites, or tartrazine, check with your pharmacist before you take any drug (both brand name and generic) for the first time.
Why do prescription drugs have more than one name?
Whether your prescription drug is a brand name version or a generic version, it will always have more than one name: a brand name and a generic name. The brand name is chosen by the manufacturer selling the brand name drug. The generic name is the name of the active ingredient and is always the same no matter which version of the drug you use.
Sometimes brand names can be confusing. It's a bit like brand names for other products. For example, you may buy Kleenex, Scotties, or Royale (brand names) but they are all facial tissue (generic name). Kleenex is a popular brand and some people may say 'Kleenex' (brand name) when they really mean 'facial tissue' (generic name).

Download a PDF copy of this FAQ here (108 KB)
Download a PDF of the poster at the top here (1 MB)
Listen to radio spots on Generic vs. Brand Name drugs: